This data was presented during the 11 February 2021 poster session of the 17th WORLDSymposium (virtual).
Introduction
Metachromatic leukodystrophy (MLD) is a rare, fatal autosomal-recessive genetic disorder caused by insufficient activity of the enzyme Arylsulfatase A (ARSA) that results in intra-lysosomal accumulation of the ARSA substrate galactosylceramide I3-sulfate (sulfatide), inevitably leading to progressive demyelination and neurodegeneration in the CNS and PNS. There are three variants of MLD commonly described in the literature based on the age at which symptoms appear: late-infantile MLD, juvenile MLD, and adult MLD. All forms of MLD share the same underlying pathophysiology and are progressive; ultimately affecting both intellectual and motor functions. Children affected by MLD display progressive neurologic symptoms, including ataxia, seizures, and quadriplegia, culminating in severe disability and early death. MLD diagnosis is often delayed or missed. To detect patients early, newborn screening is essential. The true incidence rate of MLD is unknown but is estimated to be between 1 in 40,000 and 1 in 160,000 live births. In December 2020, OTL-200 has been approved in Europe for the treatment of children with late infantile or early juvenile forms of the disease who have not yet developed symptoms and those with early juvenile MLD who have initial symptoms but can still walk independently and have not yet developed mental deterioration [1].
We have initiated a prospective newborn screening study with the implementation of MLD into the current newborn screening panel (covering 15 different diseases) for all newborns in the German states of Hamburg, Bremen, Schleswig-Holstein, and Northern Lower Saxony. The total birth rate in this area is approximately 55,000 live births per year. A tiered screening approach using tandem mass spectrometry (MS/MS) is being applied where sulfatide levels are measured in dried blood spots (DBS), followed by ARSA activity and finally genetic confirmatory testing using next-generation sequencing (NGS) (ARSA, SUMF1, and PSAP genes). For the initial validation, 200-300 samples are being analyzed to establish all necessary assay performance characteristics. Cut-off determination for primary diagnostics will be established in a pre-pilot study with circa 5,000 samples. The planned study duration is an initial 12 months with an extension for another one to two years.
Diagnosis Methodologies for MLD
1st tier testing: sulfatide profiling [2]
Determination of specific biomarker C16:0- & C16:0-OH sulfatides
2nd tier testing: ARSA activity [3]
Determination of Arylsulfatase A (ARSA) activity towards artificial substrate (GelbChem)
NGS for clinical assessment
All samples with abnormal sulfatide concentration and low/no ARSA activity are genetically confirmed by NGS
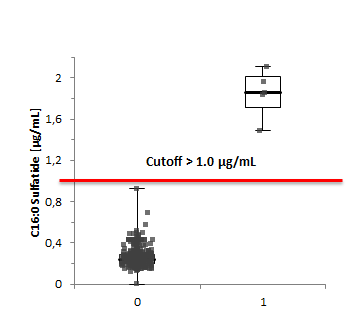
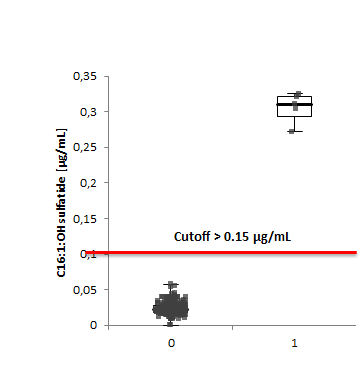
During the pilot study in 2020, 500 samples were initially investigated, and assay characteristics were established (see table below).
| Test | Criteria | C16:0 Sulfatide |
|---|---|---|
| Carry Over | < Error limit 3xSDlow-low | PASS |
| Cross Contamination | <Error limit 3xSD | PASS |
| Linearity | Optical control | PASS |
| Highest concentration for linearity experiment [µg/mL] | 1,13 | |
| LOD [µg/mL] | 0,06 | |
| LLOQ [µg/mL] | 0,10 | |
| Repeatability QC I & II (biological replicates; 10 times) | <25% | 4% |
| Reproducability (duplicates, 5 days) | <25% | 7% |
Lack of Commercial Assays and Quality Controls
- Several methods have been published (HPLC, different biomarkers, ARSA activity)
- often limited due to stability issues or technical facts such as chromatographic separation of related biomarker
- Only in-house methods are currently available (laboratory-developed test)
- No certified kit for in vitro diagnostic use according to medical product regulation (Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices).
- No Quality control materials for QC monitoring.
- No Proficiency Testing (PT) programs.
Routine Diagnostic Laboratory Requirements for Medical Reporting
- Quality control materials are necessary including precise and accurate MLD testing.
- Calibrator is not available -> Preparation “in-house” -> external calibrator (matrix-free)
- Qualified instruments (Clinical Mass Spectrometers) according to regulation (medical devices).
- Software for data calculation and reporting has to be fully validated so test results can be used for routine diagnostics and reporting to physicians.
- Proficiency testing (optional if not available: inter-laboratory testing)
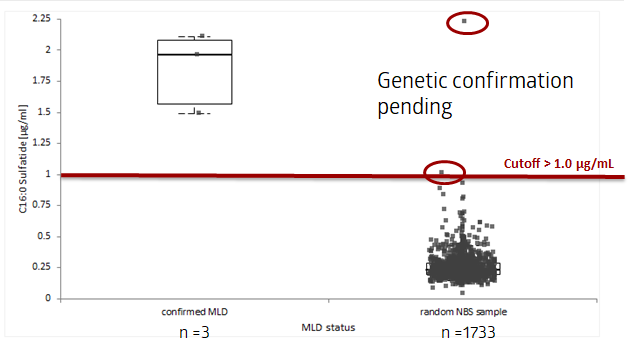
Results to date
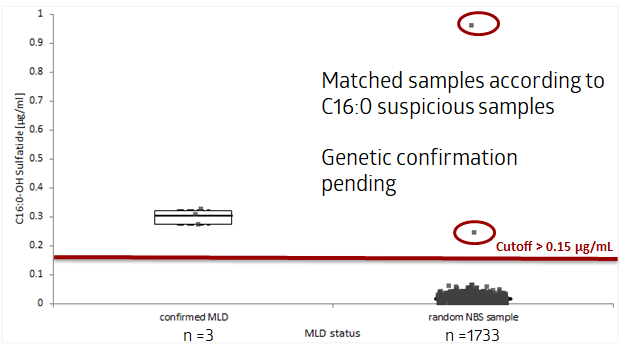
Summary
We have presented work highlighting some solutions to the diagnostic challenges for newborn screening programs. There are still financial and ethical challenges that will need to be addressed as more therapies and/or orphan drugs are being introduced for these rare diseases. Advanced diagnostics are an important part of the process because they can provide better information on the disease history and demonstrate the need for faster integration of analysis into newborn screening programs.
References
[1] Orchard Therapeutics, Libmeldy SmPC, available at: https://www.ema.europa.eu/en/medicines/human/EPAR/libmeldy#product-information-section, January 2021
[2] Hong et. al.; Genet Med 22, 1262–1268 (2020)
[3] Hong et. al.; Anal. Chem. 2020, 92, 9, 6341–6348
Authors
Markus Schwarz1, Petra Oliva1, Tom Mechtler1, Berthold Streubel2, Charlotte Chanson3, Mirko M. Essing3, Rene Santer4, David Kasper1
- ARCHIMED Life Science GmbH, Vienna, Austria
- Medical University of Vienna, Department of Pathology, Vienna, Austria
- Orchard Therapeutics (Europe) Ltd., 108 Cannon Street, London EC4N 6EU, United Kingdom
- Newborn Screening and Metabolic Diagnostics Unit, Hamburg University Medical Center, Hamburg, Germany